Abstract
Introduction: Targeted therapies inhibiting Bruton Tyrosine Kinase (BTK) (ibrutinib, acalabrutinib, and zanubrutinib) and Bcl-2 (venetoclax) have improved survival when given first line (1L) to patients with chronic lymphocytic leukemia (CLL). However, no head-to-head trials of 1L targeted therapies exist to guide clinicians on selection of optimal therapy for their patients. We performed a systematic review and network meta-analysis (NMA) to understand the relative efficacy and toxicity of 1L CLL regimens, including newer regimens of ibrutinib/venetoclax (IV) and zanubrutinib not previously assessed in NMA.
Methods: We performed a systematic literature search from 2009-2022 for randomized trials of treatment-naïve adults with CLL who were deemed unfit due to age or comorbidities. Endpoints were progression free survival (PFS) at 24 and 48 months, overall response rate (ORR), percentage of patients with grade 3 or higher adverse event (AE), discontinuation due to AE, and rates of AEs of clinical interest. We used a Bayesian hierarchical random-effects models for mixed multiple treatment comparisons to estimate the relative treatment effect of each intervention compared with a common comparator.
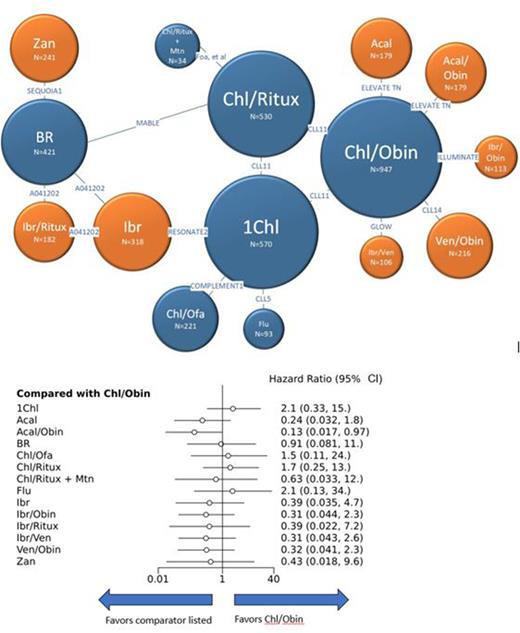
Results: Our search yielded 12 clinical trials including 15 unique 1L regimens (Fig 1). PFS and ORR of common comparators were consistent across trials except 24-month PFS for chlorambucil/obinutuzumab (CO) which ranged from 31%-64% likely due to variable duration of chlorambucil and variable inclusion of patients with del(17p). Compared to CO, only acalabrutinib/obinutuzumab (AO) showed statistically significant improvement in 24-month PFS with hazard ratio (HR) 0.13 and 95% confidence interval (CI) 0.017-0.97 (Fig 1). In general, targeted therapies trended towards longer PFS compared to chemotherapy-based regimens, though there is insufficient power to demonstrate statistical significance. We found no statistically significant differences in 24-month PFS, 48-month PFS, or ORR between targeted therapies.
Regarding toxicity, no statistically significant differences in proportion of patients experiencing grade 3 or higher AE were found across regimens, though single agent acalabrutinib and zanubrutinib trended toward less toxicity than CO with risk ratio (RR) 0.71, 95% CI 0.45-1.1 and RR 0.66, 95% CI 0.30-1.5, respectively. No statistically significant differences in discontinuation rates were found, though IV trended towards higher discontinuation rate than CO (RR 6.1, 95% CI 0.71-74). There were no statistically significant differences in rates of atrial fibrillation, infection, anemia, or neutropenia. However, grade 3 or higher thrombocytopenia was significantly lower with zanubrutinib than CO (RR 0.069, 95% CI 0.0048-0.92).
Conclusions: Using NMA, we demonstrate that PFS is improved from the pre-targeted therapy standard of care by AO, but, amongst targeted therapies, no significant differences in efficacy were identified. Our results differ from previously published NMAs which have shown superior PFS with AO compared to other targeted therapies, likely due to inclusion of additional trials of common comparators (Foa et al, CLL5) as well as newer trials assessing IV and zanubrutinib (GLOW, SEQUOIA cohort 1) in this analysis. All four trials add heterogeneity to common comparator outcomes, resulting in widened confidence intervals and loss of statistical significance. This is the first assessment, to our knowledge, of relative rates of specific AEs, and we found that zanubrutinib showed lower rates of thrombocytopenia than pre-targeted therapy standard of care. Establishing the absence of significant difference in efficacy of frontline targeted therapies in CLL allows the clinician and patient to select therapy based on other factors, namely planned duration, toxicity profile, medication interactions, and cost.
Figure 1. 24-month PFS Network and Forest Plot
1Chl = Chlorambucil; Acal = Acalabrutinib; Acal/Obin = Acalabrutinib/Obinutuzumab; BR = Bendamustine/Rituximab; Chl/Ofa = Chlorambucil/Ofatumumab; Chl/Ritux = Chlorambucil/Rituximab; Chl/Ritux + Mtn = Chlorambucil/Rituximab + maintenance Rituximab; Flu = Fludarabine; Ibr = Ibrutinib; Ibr/Obin = Ibrutinib/Obinutuzumab; Ibr/Ritux = Ibrutinib/Rituximab; Ibr/Ven = Ibrutinib/Venetoclax; Ven/Obin = Venetoclax/Obinutuzumab; Zan = Zanubrutinib
Disclosures
Klein:Lilly: Consultancy, Other: Member of Data Safety Monitoring Board; Millennium: Consultancy, Other: Member of Data Safety Monitoring Board; Pfizer: Consultancy, Other: Member of Data Safety Monitoring Board.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal